2022 NMD4C Year in Review
The NMD4C would like to reflect on all the inspiring progress the network has made towards the network’s goals in 2022. This year in review outlines a collection of achievements from the network over the past year.
Thank you to our steering committee, our working group members and leaders, patient partners, and our partner Muscular Dystrophy Canada who are an integral part of every working group!
Membership:
The NMD4C membership grew to 510 in 2022, with members from regions across Canada.
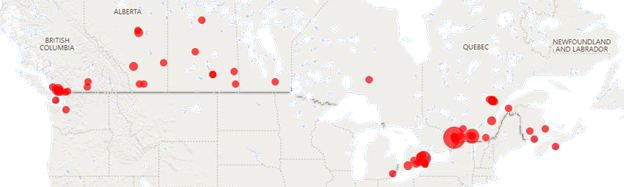
Heatmap showing NMD4C member distribution across Canada.
NMD4C Webinars:
The NMD4C continued to host monthly CPD-accredited webinars in 2022; providing 10 hours of neuromuscular training on genomics, clinical and research updates, and case-based rounds. Find a list of all our past webinars, as well as those to come in 2023 on our events page.
Biobank:
The NMD4C biobank team hired biobank developer Kevin LaFleur in August of 2022. As a research software developer for the NMD4C and the Montreal Neurological Institute, Kevin works with Dr. Jason Karamchandani to develop the technical infrastructure for the NMD4C’s centralized Canadian neuromuscular biobanking catalogue. Kevin’s work involves linking biospecimen data, imaging and genetic information from neuromuscular biobanks across Canada to a central repository within the Neuro’s C-BIG project, a leader in the open science field. The virtual biobank catalogue portal will be launching in 2023.
Clinical Trials:
In 2022, the clinical trial coordination group continued its work to unite stakeholders in the Canadian neuromuscular clinical trial landscape. Bonnie Wooten, clinical trial concierge, continued to act as an information broker for clinical trials for all Canadian clinical care sites and to support the NMD4C community with consistent knowledge, awareness, and access to information across sites. Bonnie receives between 2-6 requests each week for information on clinical trials from neuromuscular stakeholders, and curates a listing of the trials she is involved in on the NMD4C website.
Together in 2022, the clinical trial coordination team engaged with several pharmaceutical companies to address barriers to bringing neuromuscular clinical trials to Canada and readiness for Canadian sites to conduct clinical trials. We are hopeful these interactions will lead to the inclusion of further Canadian sites in future trials. The results of these meetings as well as current clinical trial opportunities were communicated to the clinical members of the network via our first clinical trial newsletter.
The clinical trial coordination team also launched a quality improvement and performance metrics survey in partnership with MICYRN to measure and collect data on industry-sponsored and investigator-initiated trials. The information gathered will help to support and streamline clinical trial processes and systems to address key barriers to conducting clinical trials in Canada.
Curriculum Development:
In collaboration with the Canadian fellowship programs, the curriculum group created a comprehensive clinical neuromuscular curriculum for fellows, with 41 topics each delivered weekly in a 1-hour lecture by a subject matter expert. This “National Neuromuscular Lecture Series” is CPD-accredited, and is accompanied by a novel training platform, where fellows can access lecture recordings and other resources. This program began in August, 2022 and the inaugural cohort will finish in June of 2023.
In the spring, the Royal College of Physicians and Surgeons of Canada approved an application for neuromuscular medicine to be recognized as an area of focused competency (AFC) discipline. Prior to the approval of this AFC discipline, no dedicated credential focused solely on neuromuscular medicine in Canada. The AFC discipline in neuromuscular medicine establishes a national standard for training and specialist competence, provides neuromuscular fellows with additional opportunities to acquire nationally and internationally portable credentials, and will help to centralize specialty training, care, and practice by providing credentials in the highly focused area of neuromuscular practice.
The curriculum development team has also hosted quarterly Neuromuscular Mystery Case Rounds, moderated by Drs. Hernan Gonorazky, Aaron Izenberg, and Christen Shoesmith. See our events page to register for the next case rounds!
Early-Career:
As a part of our annual fellowship funding competition in partnership with MDC, the early career working group awarded funding to 3 clinical and 3 postdoctoral fellowships with a total funding amount of $360 000 in 2022. Thanks to the generous supporters of MDC for this funding, as well as the Canadian Society of Clinical Neurophysiologists (who provided funding for one clinical fellowship).
The working group also produced a three-part video series called “Meet the Fellows” to learn more about the 2022 fellowship recipients!
The early-career working group launched the network’s inaugural early-career awards in 2022 to recognize achievements in the neuromuscular field through four award categories including Researcher of the Year in both clinical and biomedical streams, and Publication of the Year in both clinical and biomedical streams. These awards were developed to provide recognition to outstanding early-career investigators in the field of neuromuscular disease and an award which can be used to strengthen recipients’ academic CVs. The awards are currently in the selection process, with the recipients to be announced in March of 2023!
Expert Patient Capacity Building:
In January 2022, The Expert Patient Capacity Building (EPCB) team published a library of patient-oriented research resources on NMD4C website. This collection of online resources and websites was sourced primarily from organizations and centres across Canada but includes some from across the world and are for all stakeholders engaged in patient-oriented research (POR), including people affected by NMDs, researchers, and advocates.
The team hosted a POR webinar to provide education and training on the POR approach, how to consider key elements in developing a patient engagement plan for an NMD project, and where to locate important resources and training to support a POR project.
Speakers included patient partners Corinne Kagan and Linda Niksic, EPCB coordinator Patricia Mortenson, and investigators Dr. Cynthia Gagnon and Dr. Kathryn Selby.
Throughout 2022 the team has been working to develop an online patient-engagement training platform called “imPORTND”, created to help scientists engage with patient partners in research. imPORTND offers self-guided learning modules for research team members and patient partners to learn about patient-oriented research, determine their readiness, understand the accessibility needs of individuals living with NMD when partnering in research, and build healthy partnerships. The modules have been co-developed with a team of expert patient partners, clinicians, and researchers, and will be the first POR training that is focused specifically on neuromuscular disease. The training program is currently being translated into French to ensure that all Canadians can access the training and will launch later in February.
Knowledge Translation:
The Knowledge Translation (KT) team prepared two infographics on Clinical Care Guidelines in neuromuscular disease. The first is an infographic on the Myotonic Dystrophy Foundation’s “Consensus-based Care Recommendations for Children with Myotonic Dystrophy Type 1”, with the contribution of NMD4C investigator Dr. Craig Campbell. The second infographic is from Groupe de recherche interdisciplinaire sur les maladies neuromusculaires (GRIMN)’s “Promoting Sexuality and Intimate Relationships in Adults with a Neuromuscular Disease in Occupational Therapy”, with the contribution of Samar Muslemani, O.T., M.Sc., and former NMD4C communications coordinator. Both of these infographic share the key clinical points to consider and offer a direct link to download the complete guideline.
In 2022, the KT team produced a three-part webinar series to provide an overview of KT plan implementation from conception to execution. The series experience and recruits talented KT experts as presenters throughout the three webinars. The recordings are available to view on the NMD4C website.
The KT team also presented a poster titled “A Network’s Approach to Adapting Knowledge Translation Strategies for Neuromuscular Diseases” at the 2022 Knowledge Translation Canada Scientific Meeting.
Patient Registries:
In collaboration with the Canadian Neuromuscular Disease Registry (CNDR), the NMD4C helped launch a new dataset for Congenital myasthenic syndromes in March 2022. This initiative was led by Dr. Lochmuller and was developed in alignment with EURO-NMD. A Canadian steering committee was also created to advise on the creation and launch of the dataset. Read the full article on the launch.
Meeting in Vancouver:
The NMD4C wrapped up its 3rd Annual Investigator meeting on Tuesday December 6th, 2022 in Vancouver, BC. This hybrid meeting served as an opportunity for the network’s investigators to come together and discuss the progress made towards the network’s goals over the past 12 months, with the leaders of the seven working groups presenting the results of their work.

NMD4C investigators meet in Vancouver at the annual investigator meeting.
Thank you to everyone who has been involved in any of the network’s initiatives in 2022; the network would truly not be where it is today without all of the hard work of its members – we are excited and grateful to have the opportunity to continue working towards the network’s goals in 2023.








