Biobanking Working Group Update
NMD4C is committed to strengthening the biobanking resources available to Canadian neuromuscular researchers. In rare diseases, the availability of biosamples is often limited, which hinders research into disease pathogenesis and mechanisms, and therapy development.
NMD4C is pleased to announce the addition of network collaborator Dr. Jason Karamchandani to the biobanking working group. Dr. Karamchandani is an Associate Professor in the Department of Pathology and a neuropathologist at the Montreal Neurological Institute and Hospital, who brings biobanking experience from his work with the Clinical Biospecimen Imaging and Genetic Repository (C-BIG). Dr. Karamchandani will lead the NMD4C in creating a virtual Canadian biobank, a central catalogue which will contain the neuromuscular samples available for research at the different biobanking sites across Canada.
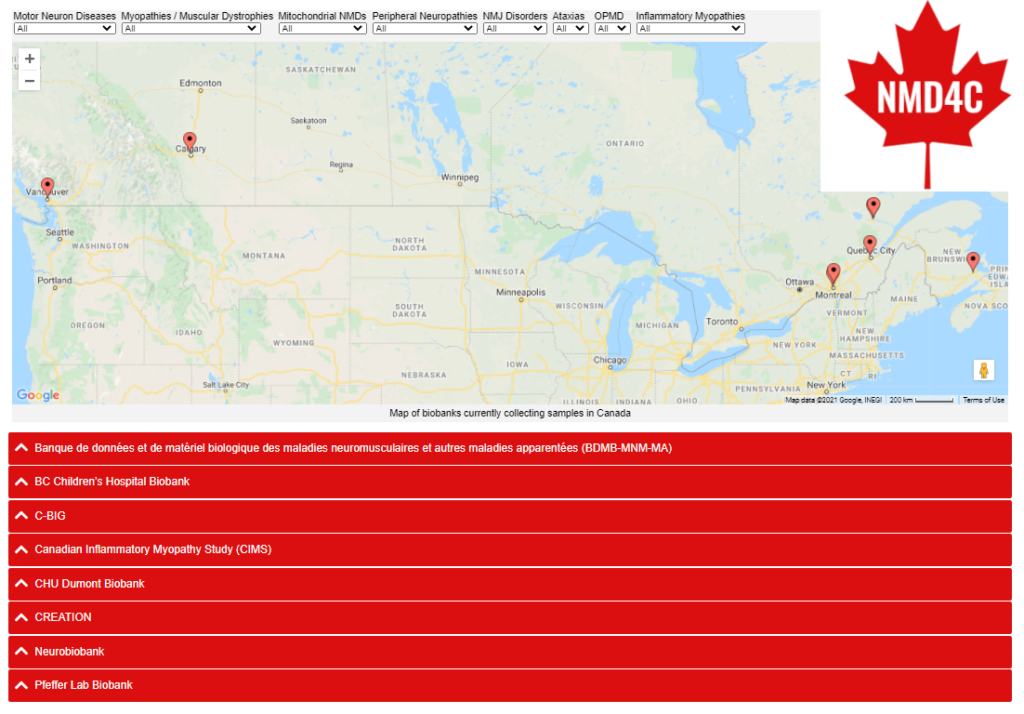
The NMD4C biobanking group has identified eight biobanks (BDMB-MNM-MA, C-BIG, CHU Dumont Biobank, CIMS, CREATION, Neurobiobank, Pfeffer Lab Biobank) that collect and prepare a variety of samples covering a range of neuromuscular diseases. Each of these sites has provided information about what they collect, and the conditions and policies for researchers to access them. While the virtual biobank catalogue is under development, we have listed the biobanks with a summary of the NMDs covered, samples collected, associated sample data, material transfer agreements in place, context of how samples are used, and contact information on the NMD4C site, which you can access here.
If you know of any biobanks that we have missed or are involved in sample collection yourself and are interested in being listed on the NMD4C site and contributing to the virtual biobank catalogue, please let us know!